
Infectious Disease
We're supporting science to bring innovative solutions to combat infectious disease in the most affected communities.
Both the Pfizer-BioNTech and Moderna Covid-19 vaccines, unlike the vaccines we already use for other diseases, have been developed using ribonucleic acid (RNA) technology. So, how do they work, are they safe, and what else do we know about them?
RNA, closely related to DNA, is present in all living cells. The strand of it called messenger RNA (mRNA) is a sequence of genetic code that tells cells what proteins to build so that they can function.
Pressing play on the video above will set a third-party cookie. Please read our cookie policy for more information.
Professor Luke O'Neill, Chair of Biochemistry at Trinity College Dublin, explains what mRNA vaccines are.
Anna Mouser: This pandemic has seen us develop a new kind of vaccine, the mRNA vaccines. I wonder if you could talk through your way of explaining how those work because they are a new type?
Professor Luke O’Neill: What it is, is the recipe to make the spike protein. So, you literally inject the recipe of the spike into your muscle and your body reads off that recipe and makes lots of spike protein. This is a viral protein and remember there is no virus there, it’s just the spike, it can’t make you sick. It’s not like getting infected.
Now you make the spike and now your immune system makes antibodies and T Cells to recognise that spike protein. So, it’s remarkably simple in a way and certainly, the Pfizer one is the first time an mRNA has been approved for use and now it’s been used in millions of people. So, it’s a great example of basic discovery I suppose giving rise to a whole new approach.
I’d also say Anna, that they decided to try it because other approaches have failed previously with things like the common cold. Now this virus as we all know is in the same family as the common cold virus. So hence there was a need for brand new technology, and that’s what this mRNA approach was.
To produce an RNA vaccine, scientists develop a synthetic version of some of the virus’ messenger RNA.
When this is injected into the human body, our cells read it as an instruction to start building the proteins, including, in this case, Covid-19's distinctive 'spike' protein.
Our bodies then mount an immune response by producing antibodies to fight the virus proteins made by our cells. This prepares our immune system to fight the real virus if we encounter it later on.
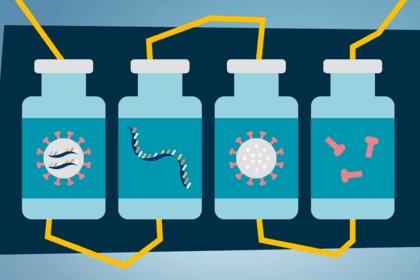
This is different to the way some other vaccines work, where a small part of the virus itself, or the whole virus (weakened or dead), is injected into the body to trigger an immune response.

A synthetic version of part of the virus' genetic code is injected. It tells our cells to start building the virus protein, triggering an immune response.
RNA vaccines hold the promise of being faster, cheaper, more adaptable and easier to mass-produce than other vaccines, because:
The Pfizer-BioNTech and Moderna vaccines are the first RNA vaccines ever to be approved for use against any disease.
However, researchers have been using the technology for a while, and people have been given RNA vaccines in clinical trials for other diseases, like cancer.
A major challenge in the past has been figuring out how to deliver the RNA vaccine into the cell so it survives – our bodies naturally want to destroy foreign RNA molecules.
This new use of RNA has only been made possible due to the enormous level of research funding and focus during the pandemic, which has allowed breakthroughs in new technologies.
The cutting-edge method could revolutionise vaccine development for existing conditions and diseases – and future outbreaks. A vaccine for HIV and therapies for cystic fibrosis and multiple sclerosis are just some of the new mRNA treatments in development.
Pressing play on the video above will set a third-party cookie. Please read our cookie policy for more information.
Voiceover: Imagine if vaccines could be created at previously unimaginable speeds. That is becoming a reality thanks to new platform technologies that can rapidly create multiple vaccines designed to protect against deadly diseases including Covid-19.
Professor Robin Shattock: So, we've been looking for technology that could make a vaccine from discovery to being in a vaccine vial within a matter of weeks.
Voiceover: When scientists detect a potentially deadly new pathogen, they can now map its genetic blueprint and synthesize small sections of it. These tiny harmless pieces of artificial genetic code are being used to create a safe, effective synthetic vaccine against the coronavirus that causes Covid-19.
Professor Robin Shattock: The manufacturing of the vaccine is going ahead so we anticipate we'll be starting our clinical trial – our first clinical trial – in June of this year.
Voiceover: That will show if the vaccine successfully protects against Covid-19.
Professor Robin Shattock: We already know that the manufacturing partners we're working with can produce five million doses within a period of weeks. Going from, you know, millions to billions of doses then becomes a global effort.
Voiceover: The new vaccine technology platform, called RapidVac, makes that much easier than before. Because each new vaccine is based on a genetic template, it can quickly be shared with vaccine manufacturers located around the world. That will allow enough vaccine doses to be equitably produced for the global population and much more quickly stop the spread of a pandemic disease. This will be particularly important for poorer countries that lack robust healthcare systems, and have large populations living in dense urban cities and slums where social distancing is impossible.
Professor Robin Shattock: I think science is absolutely critical to getting out of this pandemic.
Voiceover: In the future, vaccine platform technologies such as RapidVac will be needed to rapidly and cheaply produce vaccines that can protect against other emerging diseases.
Professor Robin Shattock: Covid-19 needs to drive the discussion so that we don't get caught out the next time.
Voiceover: Innovations like this will help us save more lives. Let's get behind the future of vaccines.
Before any vaccine can be approved for use it must go through rigorous testing, to make sure it is safe, as well as effective.
Around 43,500 people were enrolled in the Pfizer-BioNTech clinical trials, and 30,000 people in the Moderna clinical trials. Safety was assessed throughout, and no major side-effects were reported during the phase I, II and III trials.
Once the trials are complete and the full data has been analysed, regulators around the world review it and decide whether the vaccines can be approved for use in their countries. They look at all the preclinical, clinical and manufacturing process data, including the safety and efficacy data.
Once approved, the vaccines will be monitored as they are given to prioritised high-risk groups, to understand how they perform in different population groups over time, and to look for very rare side-effects or long-term safety issues. Of the billions who have received the vaccines so far, few people have had a very rare allergic reaction.
Nothing in medicine is 100% safe, and very rare side-effects may emerge as billions of people are vaccinated. This is the same for all vaccines.

We're supporting science to bring innovative solutions to combat infectious disease in the most affected communities.
Pfizer-BioNTech produced three billion doses in 2021 and aims to boost supply to four billion doses in 2022.
Moderna manufactured around 800 million doses in 2021 and plans to produce up to three billion doses in 2022.
There are now 21 Covid-19 vaccines – made using very different technologies – being rolled out in countries around the world, and hundreds of candidates in development.
Both vaccines showed efficacy of around 95% in phase III clinical trials in December 2020. This figure has changed over time, with the vaccines being rolled out and monitored in a ‘real world’ setting.
At six months after vaccination, Moderna has said its vaccine has 90% efficacy against infection and Pfizer-BioNTech has said its vaccine has 91.3% efficacy against infection.
But there are still many outstanding questions, for example how long immunity will last for, how effective the vaccines will be in different populations, whether people can still transmit the disease to others if they’ve been immunised – and how well the vaccines will work against new variants
Pfizer-BioNTech has reported high levels of vaccine efficacy in over 65 year olds – one of the groups most at risk of serious illness – which is very promising.
Although many vaccines need to be refrigerated – usually around 2 to 8C – the Pfizer-BioNTech Covid-19 vaccine needs to be kept cooler. In the initial distribution push, the vaccine needed to be stored at at least -70C. This posed problems for transporting and storing it, particularly in low- and middle-income countries where refrigeration facilities may be limited.
In May 2021, following new data shared by Pfizer, the vaccine was approved for storage in fridge temperature for up to one month, making it more widely available.
The Moderna vaccine can also be stored at fridge temperature for 30 days (2 to 8C) once delivered to healthcare facilities, but requires -20C for long-term storage and transportation.
It is critical that we continue with efforts to ensure fair access to Covid-19 vaccines, for example through the COVID-19 Vaccine Global Access Facility (COVAX). This will be instrumental to ensuring effective vaccines are prioritised for those most in need around the world.
The world needs a range of vaccines that have different characteristics, are suitable for people of all ages and ethnic groups – including people with underlying health conditions, and able to be distributed and used in all settings around the globe. They must also be available in the billions of doses. One or two vaccines won't be able to achieve this, and so we must continue to develop multiple vaccines using multiple scientific approaches to be able to control the pandemic.

Head of Prevention, Charlie Weller spoke to us about how the lessons of previous epidemics helped us respond to Covid-19.
Over the past decade we’ve seen the health and economic impact of influenza, SARS, Zika, Ebola and now Covid-19. There are certainly more outbreaks to come, but we don’t know when and where they will emerge, which makes preparation difficult. If we can hone new methods of developing vaccines, we’ll be much better prepared for any future outbreaks and able to save more lives with vaccines, faster.
That is why the urgent funding gaps in the global response to Covid-19 must be addressed. Only through appropriate funding can innovations like RNA vaccines be made possible.
We’re funding research to better understand what causes and drives infectious diseases to escalate and the solutions to control their impact.
There are currently no open funding opportunities for Infectious Disease. Learn more about the funding we provide.
This explainer was first published on 13 November 2020.
Did you find this content useful? Let us know your feedback at webmaster@wellcome.org.


Get the latest news about Wellcome and the work we fund in a monthly email.