How have Covid-19 vaccines been made quickly and safely?
Unprecedented international cooperation and focus have led to multiple effective and safe Covid-19 vaccines in less than a year, and created a blueprint for future vaccine development. Here's how.
Pressing play on the video above will set a third-party cookie. Please read our cookie policy for more information.
The first vaccines for Covid-19 were developed in under twelve months. How was this possible and what can we learn from this?
How have Covid-19 vaccines been made quickly and safely?
More people are working on vaccines than ever before. When making vaccines normally a lot of time is spent waiting for people to become infected to test the vaccine, waiting for independent agencies to check results, deciding whether to continue an expensive development process or for regulators to approve things.
Basically, waiting for things to happen one after the other, in sequence.
It's like crossing town during rush hour. You have to wait for the lights, the traffic, and the lorries stuck up a one-way street.
In developing Covid-19 vaccines, everyone is acting urgently. So it's like crossing the same town when all the traffic lights have been turned green and you've got a police escort. By cutting out all that waiting, you get there much faster but no less safely.
To make Covid-19 vaccine development faster, people signed up for trials before the vaccines were even ready. Global collaboration has led to simultaneous international testing. Regulators have been checking results on a rolling basis week by week. And money has been made available to start manufacturing before tests are finished, risking money but saving time.
All steps that can be have been carried out simultaneously.
The result has been the fastest vaccine development we've ever seen. All without cutting back on testing and safety.
Covid-19 has shown what we can achieve if we all work together, without cutting corners.
The Covid-19 pandemic threatens every one of us, wherever we are, which has demanded a new global approach to vaccine development. There has been unprecedented international attention, cooperation and use of resources, enabling us to act at speed to stop people dying and protect livelihoods.
For most diseases, developing a vaccine can take more than 10 years. The development process is expensive, so to keep costs down development takes place slowly, each stage only beginning when the previous stage is successfully completed.
This has meant a fundamental redesign of the staggered approach of conventional vaccine development, so that Covid-19 vaccine development can safely be done much faster.
So far, it has been an extraordinary success – a brilliant example of what we can achieve when we work together.
It’s a bit like driving across a busy city in rush hour. Normally you spend lots of time waiting at traffic lights, but when you have a police escort, you can take the same journey and get to the same place, just as safely, but faster.

Vaccines developed by Pfizer-BioNTech, Moderna and Oxford-AstraZeneca are the first Covid-19 vaccines to get emergency authorisation.
Pedro Vilela / Stringer / Getty Images
The usual vaccine development process
All licensed vaccines currently available have been made using a traditional vaccine development model. Because of the high costs and failure rate, this usually follows a linear sequence of steps.
There are five stages to the process:
- Discovery research – normally takes between 2 and 5 years and involves lab-based research looking to find ways to induce an immune response at a molecular level.
- The pre-clinical stage – takes up to 2 years and involves testing in animals to assess the safety and suitability of potential vaccines for humans.
- Clinical development involves testing potential vaccines in humans and has three phases:
- phase I: testing for safety – takes 2 years and requires 10-50 (usually healthy) people to take part in trials.
- phase II: understanding the immune response, safety and dosage – takes 2 to 3 years and requires hundreds of people to take part in randomised trials, including a placebo control group and people with the target disease.
- phase III: assessing if the vaccine safely protects against the disease – including prevention of infection and related immune responses – takes 5 to 10 years and requires thousands of people to take part in trials, including a placebo control group.
- Regulatory approval – can take 2 years and involves submitting data and information on the vaccine’s safety and efficacy to regulatory authorities for review, to gain approval. Pharmaceutical companies continue to monitor effectiveness and safety after the vaccine has been licensed.
- Manufacturing and delivery – require specialist facilities that are highly regulated and expensive to set up.
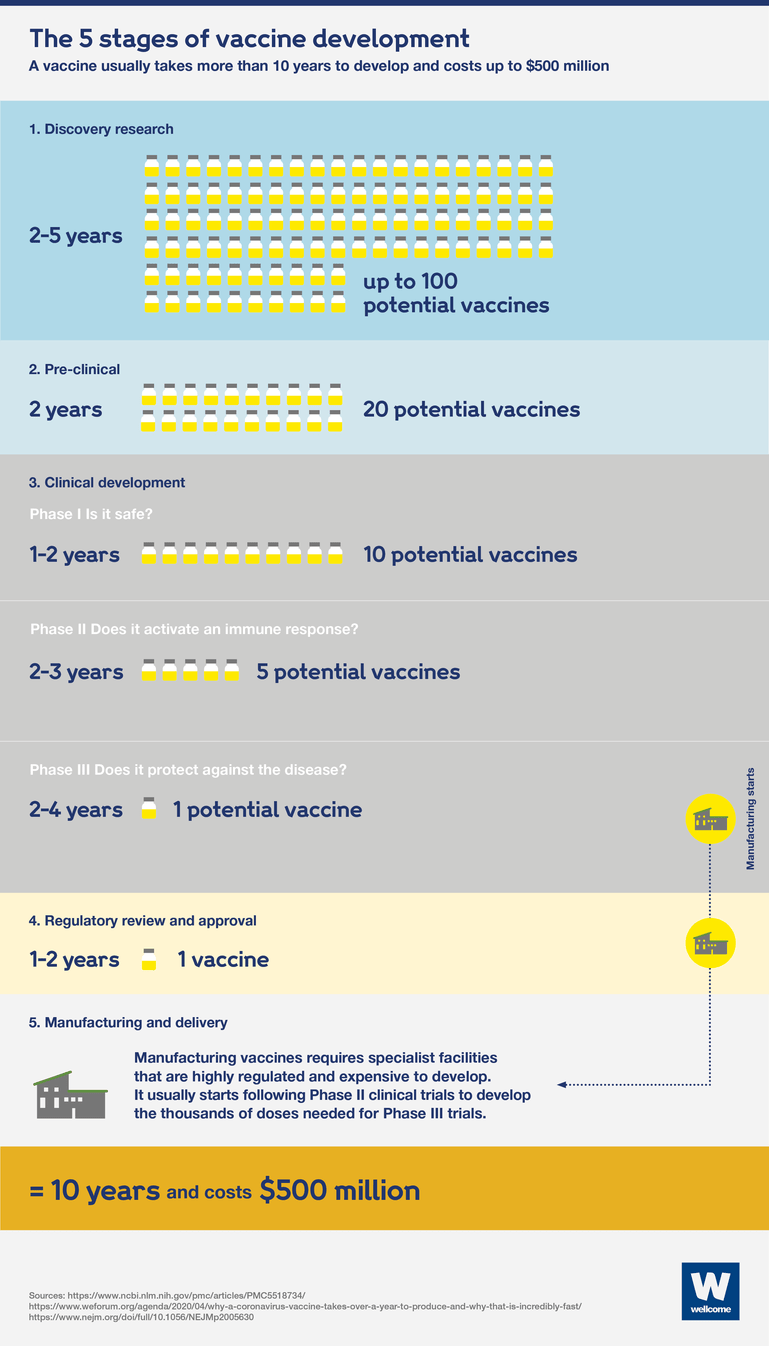
Using this approach, a vaccine would usually take more than 10 years to be developed and cost between $200 and $500 million.
Each of these stages happens in sequence, one after the other. At each stage, and between stages, there would be a lot of waiting.
With Covid-19, we couldn’t afford to wait. Because of how deadly and disruptive Covid-19 is, we simply had to find ways to speed up the usual vaccine development approach.
What changed to develop a Covid-19 vaccine at speed?
Developing Covid-19 vaccines in one year instead of 10 has been a monumental task. To succeed, new collaborative approaches to science and global manufacturing and distribution have been created.
The result has been faster vaccine development than we’ve ever seen, but without cutting back on testing and safety measures.
This has been possible thanks to public, private and philanthropic collaboration and investment on a never-before-seen level.
The investment needed for Covid-19 vaccine development is significant. $2 billion has been spent by COVAX alone, and they require a further $6.8 billion in 2021 to achieve their goal of delivering 2 billion vaccine doses globally.
While this sounds costly – at least four times the cost of usual vaccine development – it’s a good investment, given that we’re losing $375 billion from the global economy every month due to the pandemic.
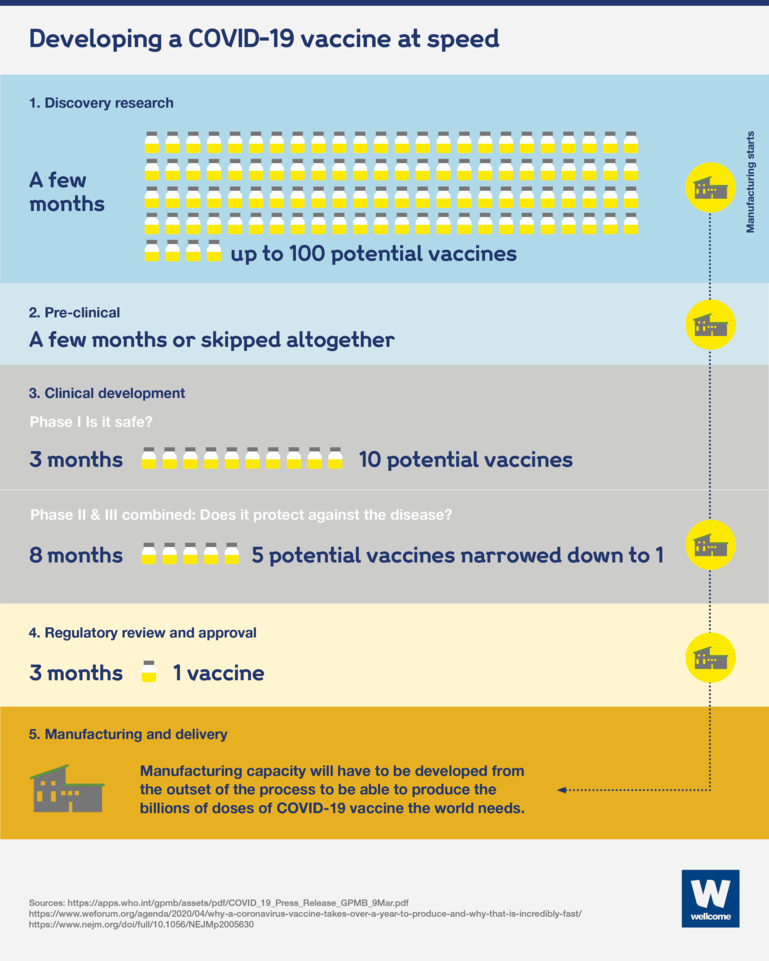
To work together at speed, researchers, developers and funders have had to seek three things:
1. Unprecedented collaboration
To work at speed has meant carrying out different stages of development and production at the same time, to get to a vaccine faster.
Vaccine trials have been carried out in parallel around the world, not just in high-income countries, to give us the best chance of finding vaccines that are safe and effective for everyone.
2. Funding for multiple vaccines
We didn’t and still don’t know where the best Covid-19 vaccines will come from, so teams are trying as many different innovations and technologies as possible. This gives us the best chance of finding ones that work, and a diversity of vaccines with different requirements to make sure they work in a variety of contexts and populations.
3. Creation of additional manufacturing capability
To meet the demand for the billions of doses of Covid-19 vaccines (in addition to all the other routine vaccines that still need to be manufactured, such as MMR and polio) requires various steps to be taken:
- manufacturing was started before the Covid-19 vaccines were proven to be safe and effective. This was done to avoid delay when a vaccine was approved, but at a risk to the vaccine manufacturers. If a vaccine wasn’t approved, they would have to bin what they had made, a bit like making a lot of food for an event that doesn’t go ahead.
- some new manufacturing sites have had to be built, and more might be needed. This is because many existing sites are still needed to produce routine immunisations which must be kept up where possible to limit the burden of additional outbreaks. Also, new sites were needed to manufacture some of the novel vaccine technologies that are being tried for Covid-19, which have not been produced at scale before.
- production sites have to be spread around the world, to help make sure vaccines are equitably distributed to communities everywhere.
- a diverse pool of vaccines is needed to get control of this pandemic, so we must continue to develop additional safe and effective vaccines, particularly ones that are easier or cheaper to manufacture and deliver. This could include vaccines that only require one dose. We will stockpile new vaccines, ready for trials and emergency authorisation for future outbreaks, beyond this pandemic.
This explainer was originally published in April 2020 and updated in January 2021.
Did you find this content useful? Let us know your feedback at webmaster@wellcome.org.