Accelerate interventions
Our work advances the discovery, development and optimisation of interventions to control and combat infectious diseases.
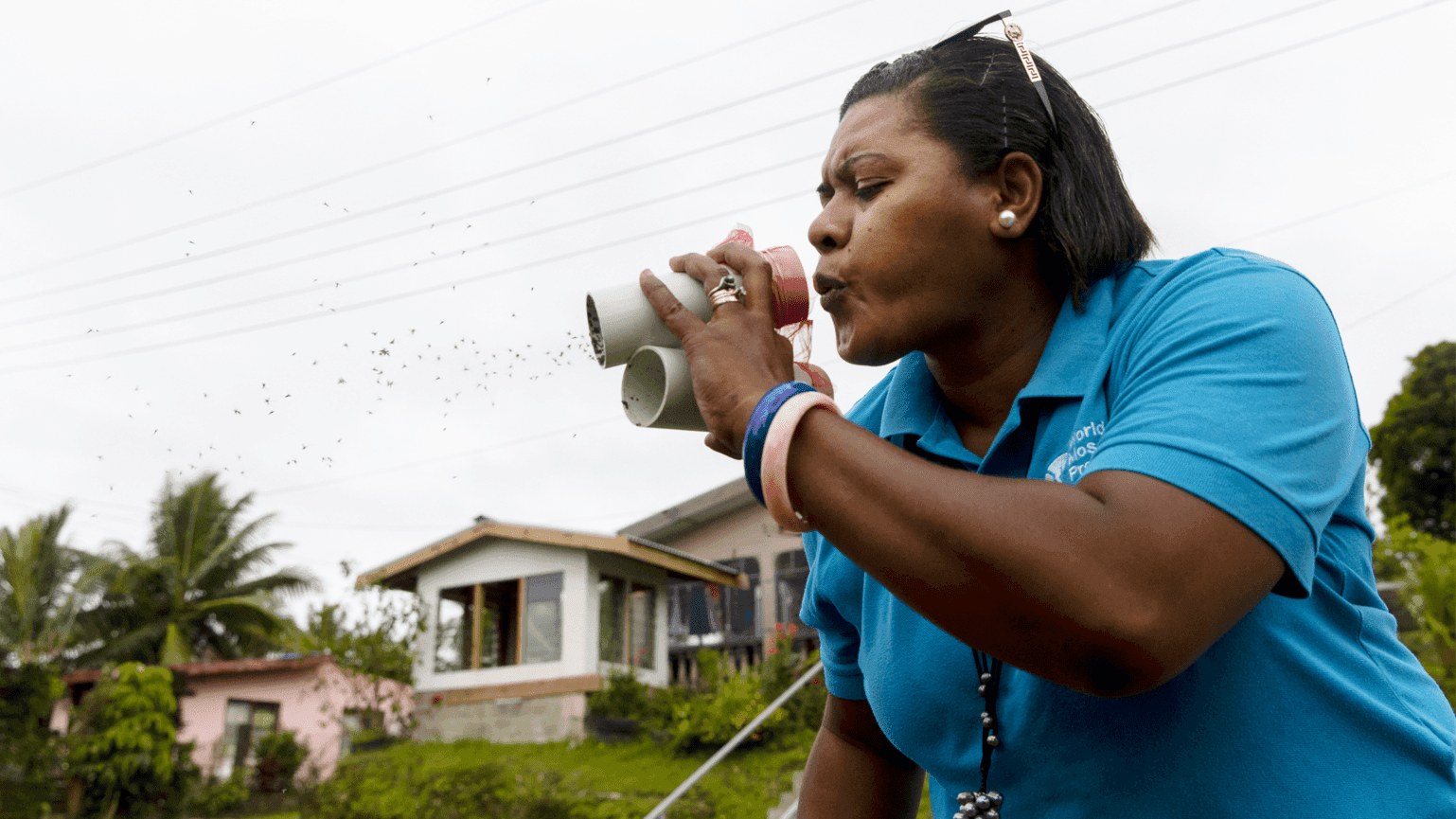
Adi Ravoka takes part in the World Mosquito Programme in the South Pacific.
Adrienne Surprenant / Collectif Item © Wellcome
Adrienne Surprenant / Collectif Item © Wellcome
Adi Ravoka takes part in the World Mosquito Programme in the South Pacific.
Our mission is to support science to bring innovative solutions to combat infectious disease in the most affected communities.
We maximise this impact through partnerships, advocacy and championing of evidence.
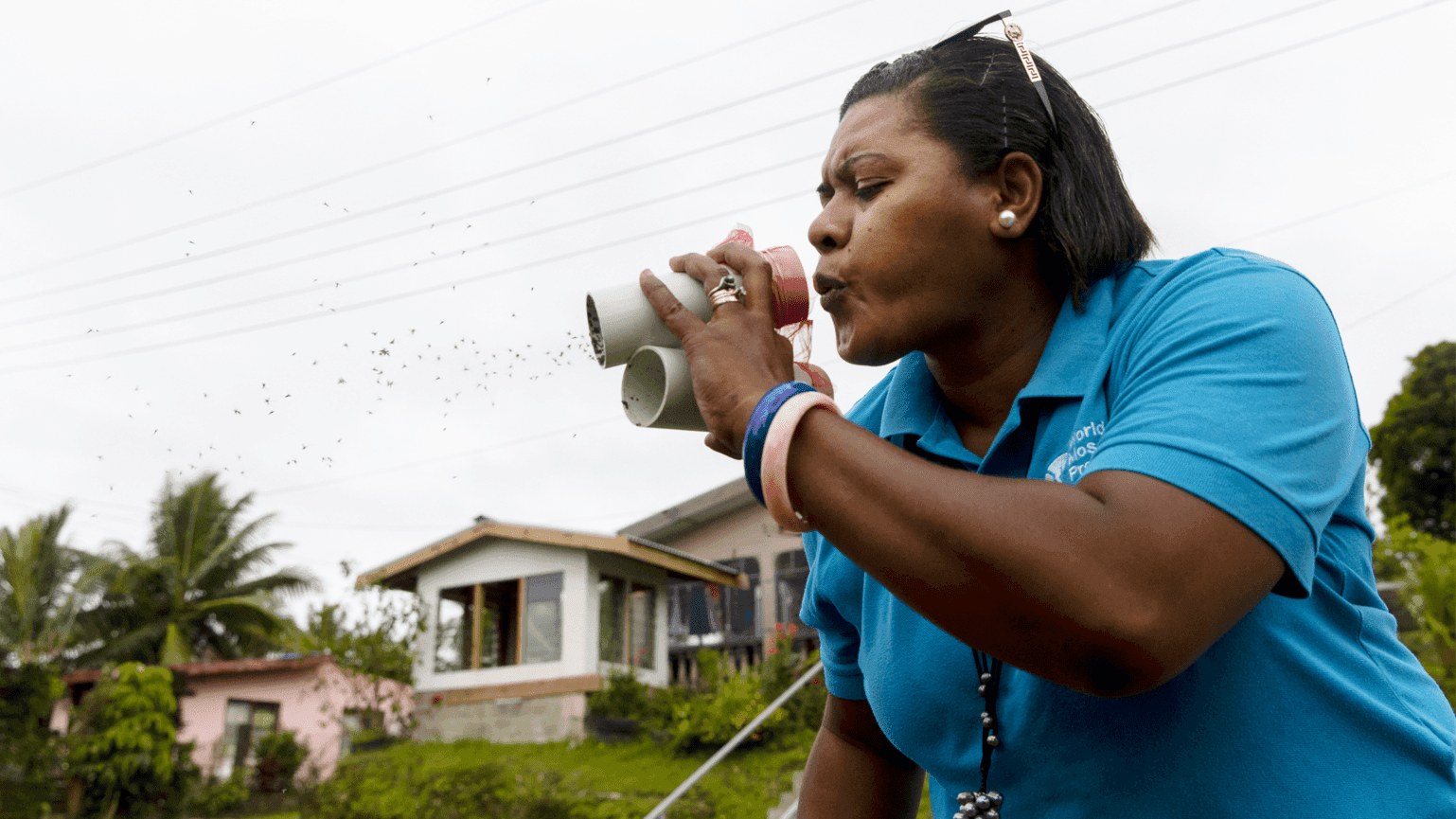
Adi Ravoka takes part in the World Mosquito Programme in the South Pacific.
Adrienne Surprenant / Collectif Item © Wellcome
Adrienne Surprenant / Collectif Item © Wellcome
Adi Ravoka takes part in the World Mosquito Programme in the South Pacific.
Infectious diseases are one of the biggest health challenges in the world today. They cause around 9.5 million deaths every year and disrupt systems across society. Environmental change and drug-resistant infections threaten to make the situation much worse.
But the impact is not equal. Infectious diseases cause the most death and illness in the world's poorest countries.
We urgently need to improve understanding and find new ways to intervene. By targeting specific research problems that others are not prioritising, we can channel our resources, skills and expertise to drive impact where it is most needed.
Our work advances the discovery, development and optimisation of interventions to control and combat infectious diseases.
Research we fund helps us to understand how and why infectious diseases emerge or escape control.

Antimicrobial resistance is one of our greatest public health threats. We need collective, evidence-based action to control the escalating burden of drug-resistant infections.
We support the research and development of innovative solutions for combating infectious disease in the regions with the greatest burden. We focus on two important threats to infectious disease control:
We’re ensuring research considers the needs and priorities of the most affected communities, so evidence and interventions are acceptable, affordable and accessible in the locations they are most needed.
Through partnerships, advocacy and championing of evidence, we’re addressing barriers in the research and development ecosystem that delay innovations reaching patients.
Our Infectious Disease programme funds research to improve understanding and advance interventions to control and combat infectious diseases. You may also be interested in our Discovery Research programme which funds fields and disciplines seeking new knowledge on life, health and wellbeing, including all aspects of infectious disease.






This policy brief identifies opportunities to drive change in how governments respond collaboratively to antimicrobial resistance (AMR). It is designed to support discussions with and between governments in the lead-up to and following the 2024 UN General Assembly.
Stay up to date with some of the biggest stories in global health, and how we're advocating to improve health for everyone.