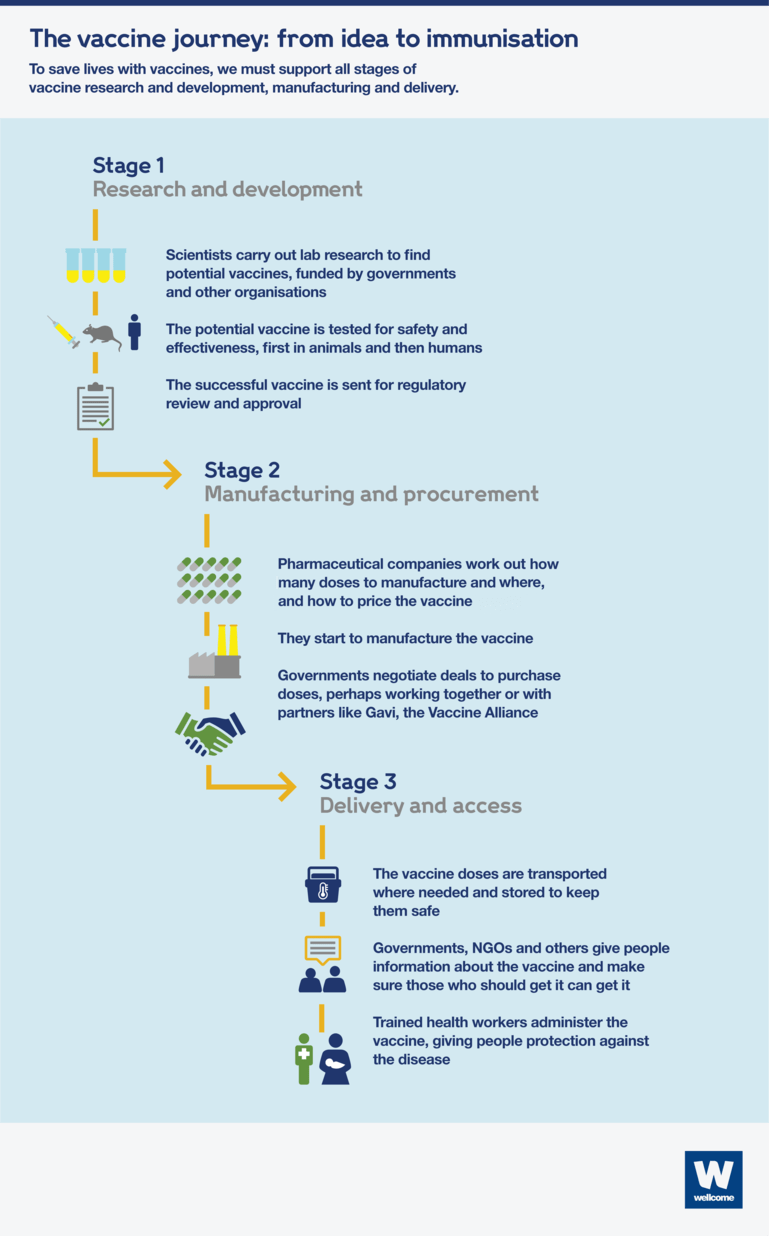
The vaccine journey: from idea to immunisation
Researchers may well identify a safe, effective vaccine for COVID-19 before too long, but all that fantastic work could count for nothing unless we also invest in manufacturing and delivery – to make sure that there is enough of the vaccine available to everyone who needs it.

Since the outbreak of the COVID-19 pandemic, we’ve seen unprecedented levels of funding for vaccine research and development (R&D). This is a great first step, but it’s only the first step.
The three main stages of the vaccine process are:
- Research and development – researchers carry out lab-based research and then run trials to test whether potential vaccines are safe and effective
- Manufacturing and procurement – companies mass-produce the vaccine and negotiate sales to governments around the world
- Delivery and access – the vaccine reaches the people who need it.
Recently, attention has focused on stage 1. But if we want to tackle an infectious disease like COVID-19 and achieve the life-saving results of vaccination, all three are essential, and all need support and funding. If one stage fails, the whole effort fails.
It’s vital that governments step up and commit investment for the whole of the vaccine process, and work with philanthropies, businesses and the global health community to drive it forward.
Stage 1: research and development
To start with, scientists carry out lab-based discovery research to understand more about the virus or bacterium – including its structure, how it enters the body, and how an immune response can be induced at a molecular level.
This research is supported by a range of organisations, including national governments, the European Commission, and funders like Wellcome and the Bill & Melinda Gates Foundation, who commit millions every year to vaccine R&D.
Once scientists find a potential vaccine, animal trials and then clinical trials on humans are needed to prove that it’s safe and that it works.
CEPI, the Coalition for Epidemic Preparedness Innovations – a global partnership between public, private, philanthropic and civil society organisations – plays a vital role in supporting, funding and coordinating clinical trials – one of the riskiest parts of vaccine development because the studies are hugely expensive and time-consuming.
CEPI prioritises vaccine development for diseases with epidemic potential. Because of this, governments have pledged millions to CEPI to stimulate research on COVID-19 around the world. Thanks to their funding and support, four possible COVID-19 vaccines are already in clinical trials.
Once a vaccine passes clinical trials, it is then submitted for regulatory review and approval. Read our explainer for a full breakdown of how vaccine R&D works.
Stage 2: manufacturing and procurement
After a vaccine is approved, manufacturing gets going.
There are a range of challenges to overcome, including:
- working out how many vaccine doses will be needed and in which countries
- finding facilities to do the manufacturing, including partners in low- and middle-income countries, to try to ensure equitable access to the vaccine
- navigating the complexities of intellectual property rights and strict manufacturing regulations that differ between countries
- setting prices for the vaccine that are affordable while also covering the R&D costs – these prices vary greatly depending on the disease and on how many doses a country can commit to buying.
Governments, as well as partners such as Gavi, work directly with manufacturers to negotiate affordable prices for buying the vaccines for many low- and middle-income countries.
Gavi also invests in vaccine stockpiles to make sure the world is prepared for future outbreaks, such as for Ebola. UNICEF works alongside Gavi by securing contracts for long-term procurement and a steady and reliable vaccine supply. This all helps to make sure that it’s not just the richest countries that have access to the life-saving vaccines when they’re needed.
Stage 3: delivery and access
Having a steady supply of vaccines at an affordable price still isn’t enough. The right infrastructure must be in place to get the vaccine to everyone who needs one.
Gavi, UNICEF, the World Health Organization and civil society organisations work with governments:
- They transport the vaccines safely to where they’re needed. This could be in the hardest-to-reach communities, so investment in roads and transport is essential. And cold-chain equipment is required to keep most vaccines cool.
- They give people information about the vaccine and make sure those who should get it can get it. Where vaccines are limited, this can also mean prioritising the most at-risk groups, such as healthcare workers.
- They train healthcare workers to administer the vaccine, giving people protection against the disease.
- In the longer term, they support population studies to check for disease outbreaks and develop secure, accurate records of citizens’ health.
The vaccine journey
How COVID-19 has changed the vaccine process
COVID-19 has required a major rethinking of almost every part of the usual vaccine process.
Research and development
- Researchers, developers and funders are working together to fund as many potential vaccine candidates as possible using a wide variety of approaches to find ones that work.
- Clinical trials are happening in sites across the world, not just in high-income countries – this will give us the best chance of finding a vaccine that is safe and effective for everyone.
Manufacturing and procurement
- Billions of doses will be required, as fast as possible. This means that global manufacturing capacity needs to be built now, and the R&D and manufacturing processes need to happen in parallel. There are risks of failure and high costs, but this approach means that, if a safe and effective vaccine is found and approved, it will be ready to distribute immediately.
Delivery and access
- There will be huge demand from countries around the world, so making sure that any new COVID-19 vaccines are first made available to those who need them most, not just those who can afford to buy them, will be crucial. Gavi, and global collaborations like the ACT Accelerator, will play a vital role in ensuring fair access to a COVID-19 vaccine, and making sure the infrastucture is in place to deliver it.
Read our explainer for more about how COVID-19 has changed the vaccine process.
However the scale and urgency of the COVID-19 pandemic don’t change one vital fact: all three stages need funding. We need to make sure we can manufacture enough doses quickly and get them to everyone who needs them.
Tomorrow’s Global Vaccine Summit is a key turning-point in making this happen, getting control over COVID-19, and ensuring access continues to the life-saving vaccines we already have.