What different types of Covid-19 vaccine are there?
There are hundreds of Covid-19 vaccines in development, and some have produced very positive results in phase III clinical trials. So, how do some of the different vaccines work and compare?

How do vaccines work?
All vaccines work by teaching our bodies to recognise and fight the pathogen in a safe way. They encourage our immune system to produce antibodies, T-cells or both, so that if we encounter the infection later our immune system knows how to defend against it.
Pressing play on the video above will set a third-party cookie. Please read our cookie policy for more information.
Professor Luke O'Neill, Chair of Biochemistry at Trinity College Dublin, explains how vaccines work.
How do vaccines work?
Anna Mouser, Policy and Advocacy Lead, Vaccines: Love to know how you explain to people how vaccines work.
Professor Luke O'Neill, Chair of Biochemistry at Trinity College Dublin: The best analogy I've used over the years is like, the bad guy arrives in town and the immune system puts up wanted posters, right, so when the real bad guy comes back and the next time he's recognised more quickly, I suppose. And in essence, what vaccines do is there are parts of – in this case, Covid-9 – the virus and then the immune system can recognise that part and then get trained to recognise it when the real bad guy arrives with those parts as well. And you make loads of antibodies to the spike. Your T cells come out, they're a very important part of the immune system as well. And they come out in numbers and then when the virus – the real virus – infects you, those antibodies and T cells are there, they've identified the virus. I call it a 'boot camp' sometimes.
Mouser: Bootcamp?
O'Neill: Yeah, there's two very important cell types: B cells and T cells. And what the vaccine does is it brings them out in numbers – from the fortress, if you will you, know. And then when you get challenged with the virus, then you have loads of troops there ready to fight the virus. And they stop you getting infected.
Vaccines can be made using many different technologies. The Covid-19 vaccines that are currently the most advanced are using four different approaches:
- viral vector
- RNA
- 'whole' virus
- protein subunit.
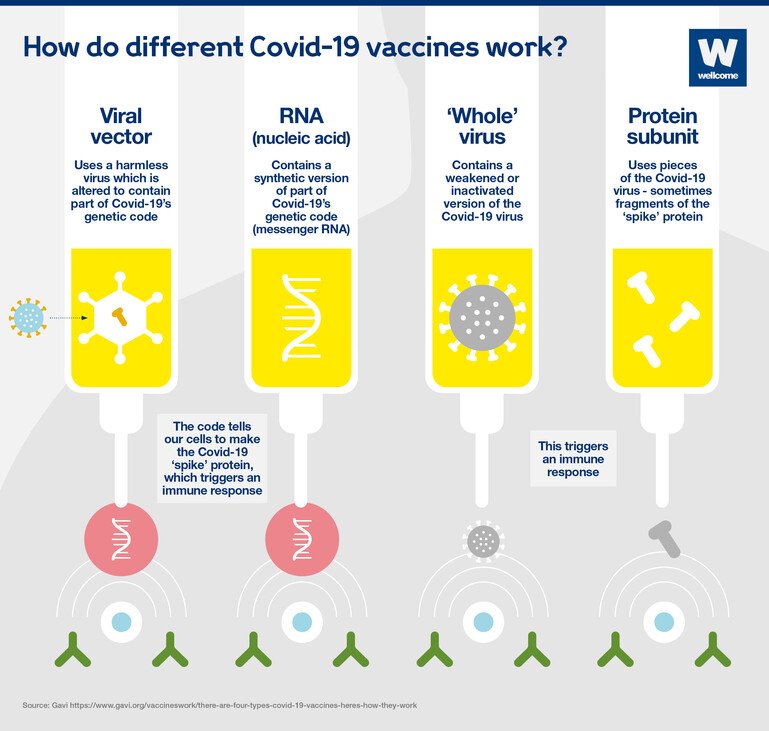
Viral vector vaccines
A harmless virus is altered by introducing part of the disease-causing virus’ genetic code, such as the code for Covid-19’s ‘spike’ protein.
The harmless virus transports the code into our cells – in a similar way to RNA vaccines – which then start to produce the protein.
This triggers an immune response, priming our immune system to attack the real virus later.
Oxford-AstraZeneca is the first viral vector vaccine to be approved for Covid-19. More are in late-stage research, such as CanSino Biologics, Gamaleya Research Institute and Johnson & Johnson. They all use adenoviruses – a group of viruses that cause the common cold – as the vector or carrier.
Another example of a viral vector vaccine is the Ervebo (rVSV-ZEBOV) Ebola vaccine – it uses the vesicular stomatitis virus as the carrier.
RNA vaccines
Messenger RNA is a sequence of genetic code which our bodies use all the time – it tells our cells what proteins to build so they can function.
To produce an RNA vaccine, scientists develop a synthetic version of the virus’ messenger RNA.
When this is injected into our bodies, cells read it as an instruction to start building the relevant viral protein, for example Covid-19's 'spike' protein. This prompts our immune system to respond, and in doing so it learns how to protect against future Covid-19 infection.
Two RNA Covid-19 vaccines have been approved for use: Pfizer-BioNTech and Moderna. Both have reported high levels of vaccine efficacy – around 95%.
They are the first RNA vaccines ever to be approved for use against any disease. However, researchers have been using the technology for a while, and people have been given RNA vaccines in clinical trials for other diseases, like cancer.
‘Whole’ virus vaccines
These vaccines could be:
- Inactivated – a version of the virus is inactivated by being exposed to heat, chemicals or radiation.
- Virus-like particle – a version of the virus, closely resembling the real thing, is created artificially, however it doesn’t contain any genetic material, so it’s not infectious.
These vaccines cannot cause the disease, but will cause our bodies to produce an immune response which will protect against future infection.
Some of the most advanced inactivated Covid-19 vaccines in development include Sinovac, Bharat Biotech and two by Sinopharm. Examples of existing inactivated vaccines include the whooping cough, rabies and hepatitis A vaccines.
One vaccine in phase III clinical trials is virus-like particle: Medicago Inc. An example of an existing vaccine is the HPV/cervical cancer vaccine.
Protein subunit vaccines
A small piece of the virus’ genetic code is inserted into another cell – perhaps a bacterial, yeast, mammalian or insect cell. The code contains instructions for this cell to start building the virus protein, for example the Covid-19 ‘spike’ protein.
Cells like this act as factories, building large quantities of the protein – which is then extracted, purified and used as the active ingredient in the vaccine.
When it is injected, our bodies learn to recognise the viral protein so that they can mount an immune response which protects against future infection.
Some of the most advanced Covid-19 vaccines using this approach include Novavax and Chinese Academy of Sciences.
An example of an existing protein subunit vaccine is for hepatitis B, which uses yeast cells to build the virus protein.
How do the different Covid-19 vaccines compare?
The different vaccine approaches present different opportunities and challenges, which is why we’ll need a range of vaccines to get control of the pandemic.
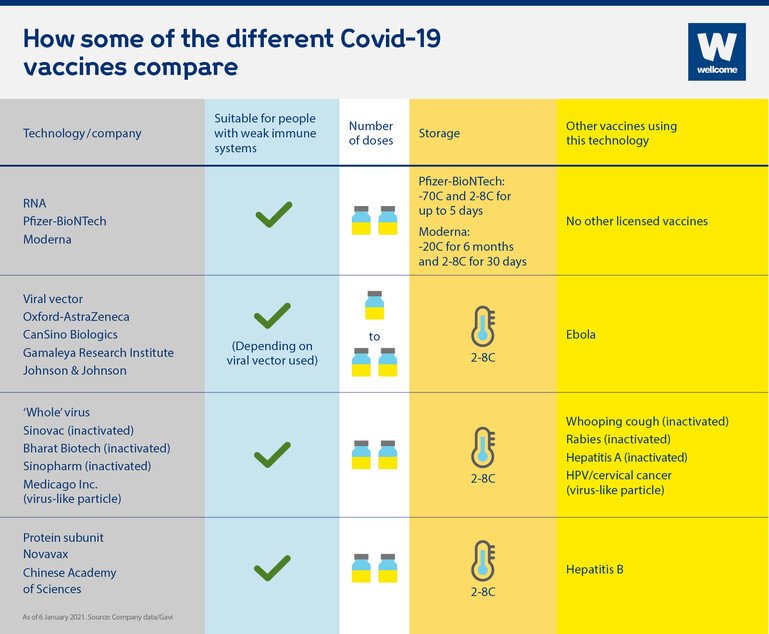
Are they suitable for people who are immunocompromised?
Although vaccines are usually safe for people who are immunocompromised, people who are severely so are sometimes advised to avoid live vaccines, because even the weakened virus can make them very sick. Encouragingly, early studies suggest that all four of the vaccine technologies currently being used are suitable for people who are immunocompromised, and as none of these technologies use 'live' virus, they should not pose a problem in this respect.
How many doses are required?
Most approved vaccines to date require two doses, spaced weeks apart to achieve full immunisation, with the exception of the single dose Johnson & Johnson viral vector vaccine.
How are they stored?
Many vaccines need to be refrigerated - usually around 2 to 8C - however RNA vaccines need to be stored at at least -70C, which could pose problems for transporting and storing it, particularly in low and middle-income countries where refrigeration facilities may be limited. The Moderna vaccine can be stored at fridge temperature for 30 days (2 to 8C) once delivered to healthcare facilities which is encouraging, but requires -20C for long-term storage and transportation.
How can we make sure that Covid-19 vaccines are accessible to everyone?
To overcome the pandemic, we have to overcome it everywhere in the world. But we will only succeed if vaccines are available and affordable to all countries.
The COVID-19 Vaccine Global Access Facility (COVAX) is working to make this happen, by ensuring that all participating countries, regardless of income levels, will have equal access to the vaccines in its portfolio once they are developed. But only three of the vaccines currently in phase III clinical trials are part of the COVAX portfolio.
There is a serious danger that low-income countries may be shut out of vaccine access, as they are less able to procure deals directly with pharmaceutical companies. For example, wealthy countries have signed deals to buy roughly 600 million doses of the Pfizer-BioNTech vaccine (which is not part of the COVAX portfolio). This accounts for nearly half of Pfizer-BioNTech's total production capacity up to the end of 2021.
So countries that have secured large numbers of vaccine doses, like the UK, must commit to donate their suitable excess doses to COVAX, so that they too can be fairly distributed.
This explainer was first published on 8 December 2020.