Infectious Disease
We're supporting science to bring innovative solutions to combat infectious disease in the most affected communities.
Momentum is growing to make sure the world is better prepared against Lassa fever. The disease is now on the priority list of the World Health Organization (WHO) and the Coalition for Epidemic Preparedness (CEPI). But we need to continue to fill in the gaps in research, as Josie Golding explains.

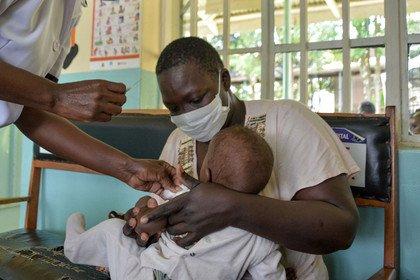
Adolphus Opera/Twenty Ten/Panos
A father and son in Bauchi, one of the regions in Nigeria affected by the 2018 Lassa fever outbreak.

Head of Epidemics and Epidemiology, Infectious Disease, Wellcome

Head of Epidemics and Epidemiology, Infectious Disease, Wellcome
Lassa fever is endemic to several West African countries, including Benin, Ghana, Guinea, Liberia, Mali, Nigeria and Sierra Leone.
In 2018 Nigeria was faced with the largest fever outbreak in the country, which proved to be one of the deadliest too, with over 140 confirmed deaths. Lassa fever normally has a fatality rate of about 1%, but in the latest outbreak it’s thought to have been more than 20% among confirmed and probable cases, according to the country's Centre for Disease Control.
The potentially fatal disease can affect many organs and damage the body's blood vessels. Most people catch Lassa fever from eating, drinking or handling anything contaminated with rat urine, faeces, blood or saliva. It can also pass from person to person through bodily fluids.
Because the symptoms are varied and non-specific – and in about 80% of the cases they do not show at all – diagnosing the disease is difficult.
There are no vaccines either, and developing them is made more difficult because the virus has multiple strains across different countries. An effective vaccine would need to protect against all of them. Treatment currently relies on one drug – but more robust assessment is needed.
To stop future outbreaks from becoming health emergencies, we need more research to develop effective vaccines, diagnostic tests and treatments.
Wellcome is funding several projects to advance research on Lassa, as part of our work on epidemic preparedness and building strong research ecosystems in Africa and Asia.
This includes funding the Centre for Infectious Disease and Research Policy (CIDRAP) to develop research and development (R&D) roadmaps for Lassa fever, Ebola/Marburg and Nipah.
These roadmaps will:
The roadmaps, which will be available in early 2019, are a crucial step in helping funders, governments and industry focus their efforts on what needs to be done next to close these gaps.
For this to be successful, coordination between all key national and international organisations is vital, as well as adequate funding and investment.

We're supporting science to bring innovative solutions to combat infectious disease in the most affected communities.
Wellcome’s global policy team has also helped the WHO R&D Blueprint team to develop a tool to map out the partners involved in the outbreak response.
With the UK Department for International Development, Wellcome is funding two projects to help with future Lassa fever outbreaks in Nigeria.