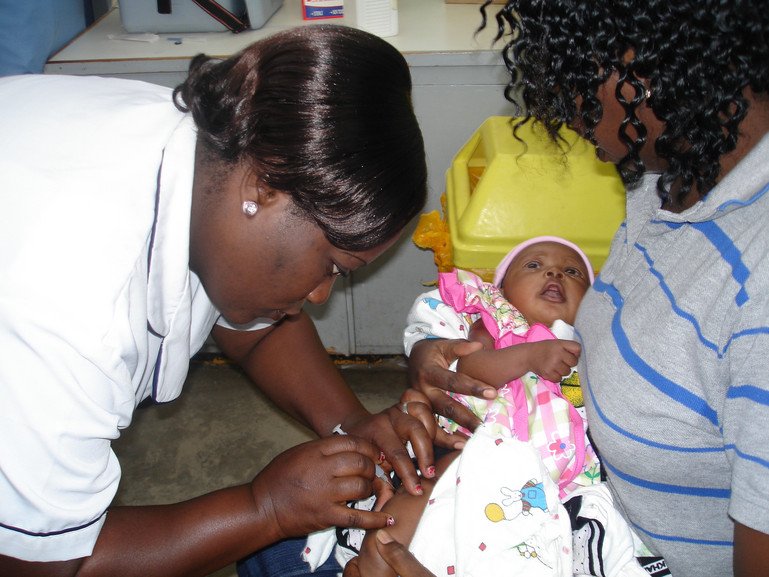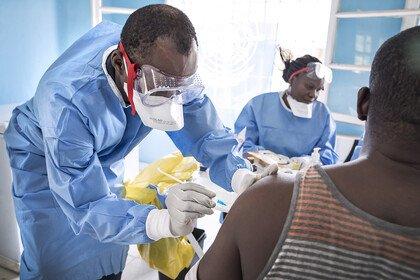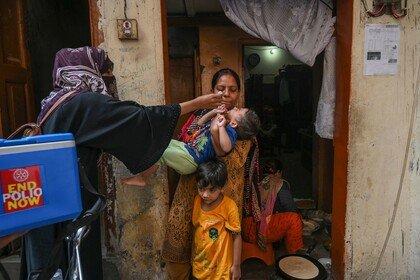
Advances in fighting malaria: from bed nets to the first-ever vaccines
The first malaria vaccine has been given to more than 1.7 million children in Africa so far and could save tens of thousands of lives when it is rolled out more widely. A second vaccine has also recently been approved for use.
We look at the history of malaria advancements and Wellcome’s involvement in recent progress.
Brian Ongoro/AFP via Getty Images
This article was first published on 28 November 2022.
In 2021, nearly half of the world's population was at risk of malaria – a life-threatening disease caused by parasites that are transmitted to humans via the bites of infected female Anopheles mosquitoes. The percentage of people at risk could increase significantly because of climate change.
Fever, chills and a headache – the first symptoms of the disease – usually appear 10 to 15 days after a person is bitten by an infected mosquito. The symptoms can be mild and difficult to recognise as malaria, sometimes until it is too late. Left untreated, P. falciparum malaria – the most deadly and prevalent parasite on the African continent – can cause severe illness and even death within just 24 hours.
According to the World Health Organization’s (WHO) latest World Malaria Report, there were an estimated 247 million malaria cases and 619,000 malaria deaths worldwide in 2021. This represents about two million more cases in 2021 compared to 2020, and 6,000 fewer deaths.
Africa carries the highest number of cases, or disease burden. These account for about 95% of all malaria cases and 96% of all deaths in 2020. Children under five made up around 80% of those deaths.
Malaria in numbers
2 vaccines approved for use
4 African countries (Nigeria, the Democratic Republic of the Congo, Tanzania and Niger) account for more than half of all malaria deaths worldwide
40% of public health budget in some African countries is spent on treating malaria
619,000+ people die from malaria each year
80% of those people are children under five
$12 billion per year in economic losses in Africa alone
(Source: World Health Organization)
Some people are at greater risk of disease. Infants, the under-fives, pregnant people, people with HIV/AIDs, and mobile populations, like migrants, who have low immunity and move into areas with high transmission, are all more likely to contract malaria, and to suffer severely.
However, it remains a largely preventable and curable disease, and several recent advancements – including the development of the first malaria vaccine – could save the lives of hundreds of thousands in the future.
How is malaria prevented and treated?
Impressive progress has been made in reducing the burden of disease – and fatalities caused by it – thanks to the development of several malaria treatments and preventions.
Antimalarials and insecticides
As early as 1820, two French chemists isolated quinine – a treatment for malaria still used today – from the bark of the cinchona tree.
Antimalarial drug chloroquine (a synthetic analogue with the same mechanism of action as quinine) and insecticide DDT were pivotal to the execution and at least initial success of the WHO’s Global Eradication of Malaria Campaign in 1955.
Research found that mosquito vectors – or carriers – of malaria rested inside houses after taking a blood meal. This made them susceptible to control through the indoor residual spraying of DDT. While it dramatically reduced transmission, the cost of spraying, and the environmental concerns about using residual insecticides, saw these programmes halted in most endemic countries that did not have the resources to continue them. When they stopped, transmission rates bounced back to the same levels as before the spraying programmes were launched.
Similarly, drug resistance to antimalarials like chloroquine that were used in mass drug administration (MDA) programmes – in which every member of a defined population is given the treatment at the same time and often intervals – impacted the efficacy of the drug.
Artemisinin and bed nets
In 1972, artemisinin was discovered by Chinese scientist Tu Youyou, who won a Nobel Prize for the discovery. Derived from the extracts of sweet wormwood plant (Artemisia annua), the drug was found to be a cheap and effective remedy for even highly drug-resistant strains of the disease. Early clinical trials on artemisinin derivatives were conducted in Thailand led by Nick White, François Nosten and Arjen Dondorp, and in Vietnam by Tran Tinh Hien and Jeremy Farrar, followed by extensive trials in Asia and Africa, including trials of artemisinin-based combination therapies.
After decades of independent randomised clinical trials and meta-analyses, artemisinin combination therapies (ACTs) were recommended by the WHO in 2006 as the first-line treatment against malaria. In the 1990s, the introduction of bed nets soaked in insecticides prevented thousands of deaths, particularly of the babies and young children who slept under them. When mosquitoes try to bite, the netting blocks them and the insecticide coating kills them. Insecticide-treated bed nets are a highly effective tool for protecting against malaria. Between 2004 and 2019, these nets averted an estimated 68% of malaria cases in sub-Saharan Africa.

A child sits under a mosquito net in his home in the town Xai Xai, Mozambique, 22 June 2005.
Alexander Joe/AFP via Getty Images
In 2009, the ACT Coartem Dispersible was developed especially for younger children by the pharmaceutical company Novartis and the nonprofit Medicines for Malaria Venture. The treatment is a sweet-tasting, cherry-flavoured tablet that disperses quickly in water, making it easier to give to young children and ensuring they receive accurate dosing. Before this breakthrough, healthcare workers and parents had to crush bitter-tasting antimalarial tablets to give to children and it was difficult to give the required dose.
Vaccines for malaria
The use of a malaria vaccine – RTS,S/AS01 (RTS,S) created by GlaxoSmithKline (GSK) – was approved for the first time by the WHO in October 2021. The vaccine is a result of 30 years of research and support from many organisations, including GSK, the Walter Reed Army Institute of Research, the PATH Malaria Vaccine Initiative, the Bill and Melinda Gates Foundation and Wellcome. So far, it has reached more than a million children in Ghana, Kenya and Malawi, where it is being piloted. It has significantly reduced life-threatening severe malaria and hospitalisation with malaria infection in these countries. Its reach will widen soon with twelve countries set to receive 18 million vaccine doses between 2023 to 2025.
What’s more is that, in a recent study part-funded by Wellcome, researchers have shown that the vaccine used in combination with antimalarial drugs can reduce malaria cases and deaths by two-thirds in children. That’s higher than either intervention given alone.
This breakthrough vaccine has the potential to save tens of thousands of lives in Africa annually. And a second vaccine, R21/Matrix-M, created by researchers from the University of Oxford provides up to 80% protection against malaria. It was approved for use by the WHO in October 2023 and has been licensed for use by drug regulators in Ghana, Nigeria and Burkina Faso.
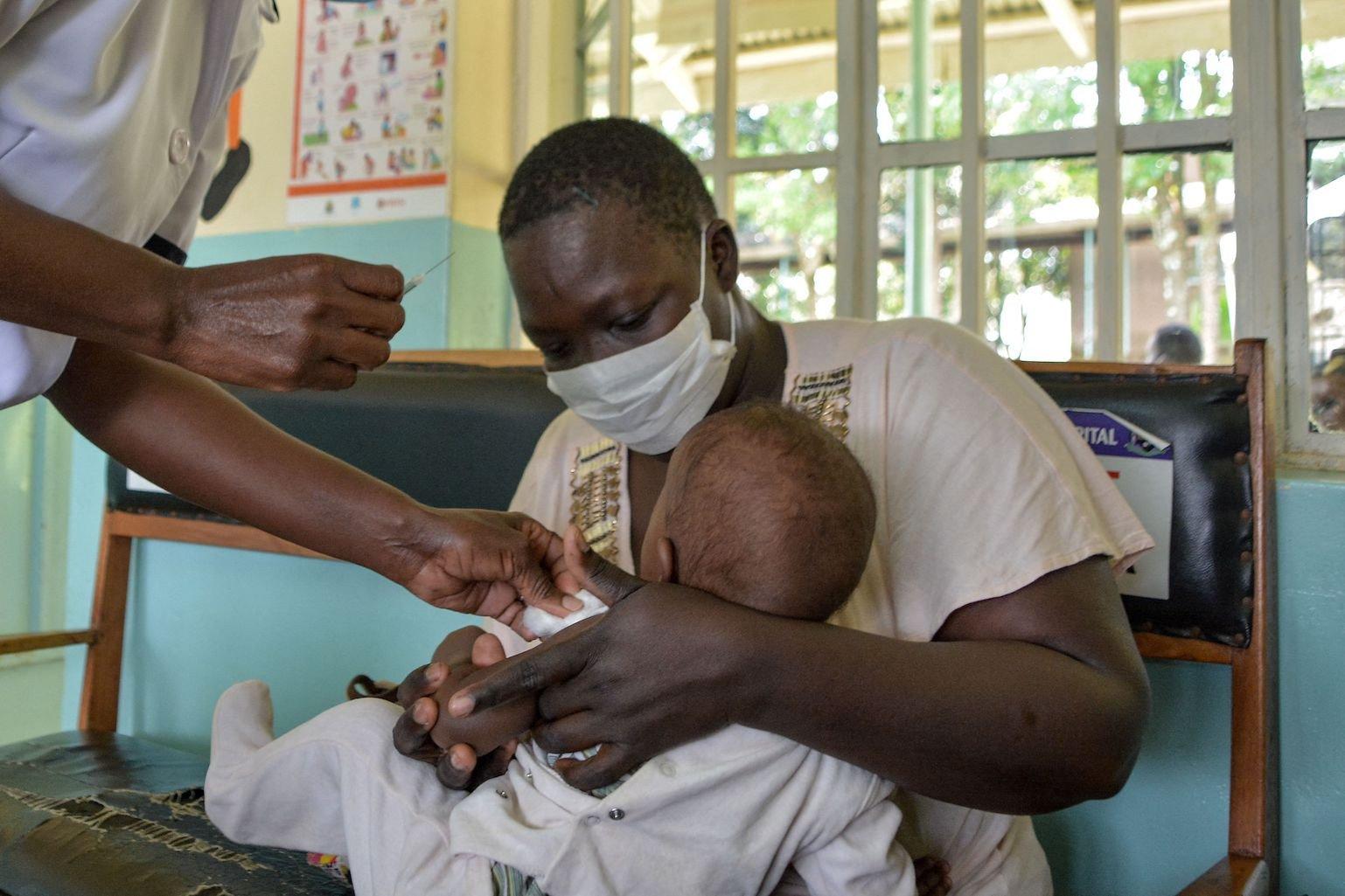
Wellcome supported the development of the RTS,S vaccine through research at the KEMRI-Wellcome Trust Research Programme in Kenya.
Stevie Mann/KEMRI-Wellcome
How is Wellcome supporting the fight against malaria?
Wellcome has supported research into malaria since we awarded our first grants to researchers back in 1938. Our funding in the UK and in countries where malaria is endemic has helped build knowledge and tools that have directly and indirectly led to advances in treatments to prevent and manage the disease. For example, we supported:
- the development of the RTS,S vaccine through research at the KEMRI-Wellcome Trust Research Programme in Kenya, and funding from the Health Innovation Challenge Fund, a partnership between Wellcome and the UK government
- large trials of artemisinin-based therapies which led to their recommendation as first-line of treatment for malaria in 2006
- trials that demonstrated the effectiveness of insecticide-treated bed nets in reducing transmission from mosquitoes to people
- research in basic biology and parasitology, as well as genetics, immunology, the history of medicine and clinical research which have all informed progress in malaria research.
Reducing the risk and impact of infectious diseases
Infectious diseases cause around a quarter of all deaths in the world – and the risk of new infections emerging is rising.
We want to ensure everyone, everywhere is protected from the threat of infectious diseases like malaria. To do that, we plan to:
- increase understanding of why diseases emerge and what drives escalation
- shift investment towards prevention and support this with tools that will help detect or predict escalating outbreaks
- and create products that are affordable, accessible and available.
A second vaccine for malaria
We’ve also part-funded the development of the new R21/Matrix-M malaria vaccine since 2017. The results of a Phase 3 trial which included 4,800 children across Burkino Faso, Kenya, Mali and Tanzania are currently under peer review. Now approved for use by the WHO, the vaccine is expected to become available to countries in 2024.
“It has taken a long time to get the R21/Matrix-M vaccine,” says Michael Chew, a Research Manager in Wellcome’s Discovery Research team. “Malaria is a complex parasite with multiple stages. It is constantly evolving. If R21/Matrix-M is rolled out to endemic populations, the impact will be spectacular in terms of mortality and morbidity, especially among children and pregnant women in resource-challenged countries.”
Pete Gardner, a Research Lead in Wellcome’s Infectious Disease team, says: “This vaccine could have a huge impact on people who live in countries where malaria is a threat. Whilst the RTS,S vaccine is a huge achievement and the first vaccine for human parasitic disease, the efficacy is still quite low.
“We need a higher efficacy and low-cost vaccine urgently. R21/Matrix-M looks very promising as an anti-disease vaccine to protect children under five who make up approximately 80% of deaths in Africa from malaria.”
Charlie Weller, Head of Prevention in Wellcome’s Infectious Disease team, adds: “The WHO recommendation for the use of the R21/Matrix-M malaria vaccine is promising progress. While further research is needed to fully understand the vaccine and its potential impact on reducing the impact of the disease, this outcome is a great example of how researchers and policymakers can work collaboratively to enhance our preparedness and prevention against escalating infectious diseases.”
The lessons learned from developing the RTS,S and the R21/Matrix-M vaccines, such as the potential of human infection studies, will not only inform future vaccines for malaria, but vaccines for other diseases and research in other fields too. At Wellcome, we believe the greatest advances in knowledge can come from unexpected places – and that’s why we’re committed to continue funding ambitious discovery research that has the potential to improve people’s health, as well as targeted funding for infectious disease research.
We’re funding research to better understand what causes and drives infectious diseases to escalate and the solutions to control their impact.
There are currently no open funding opportunities for Infectious Disease. Learn more about the funding we provide.