Landmark moment in the fight against Ebola: what have we learnt?
The licensing of the first Ebola vaccine is a great achievement for the global healthcare community. What made it possible, and what lessons can we use to prevent and control future outbreaks?
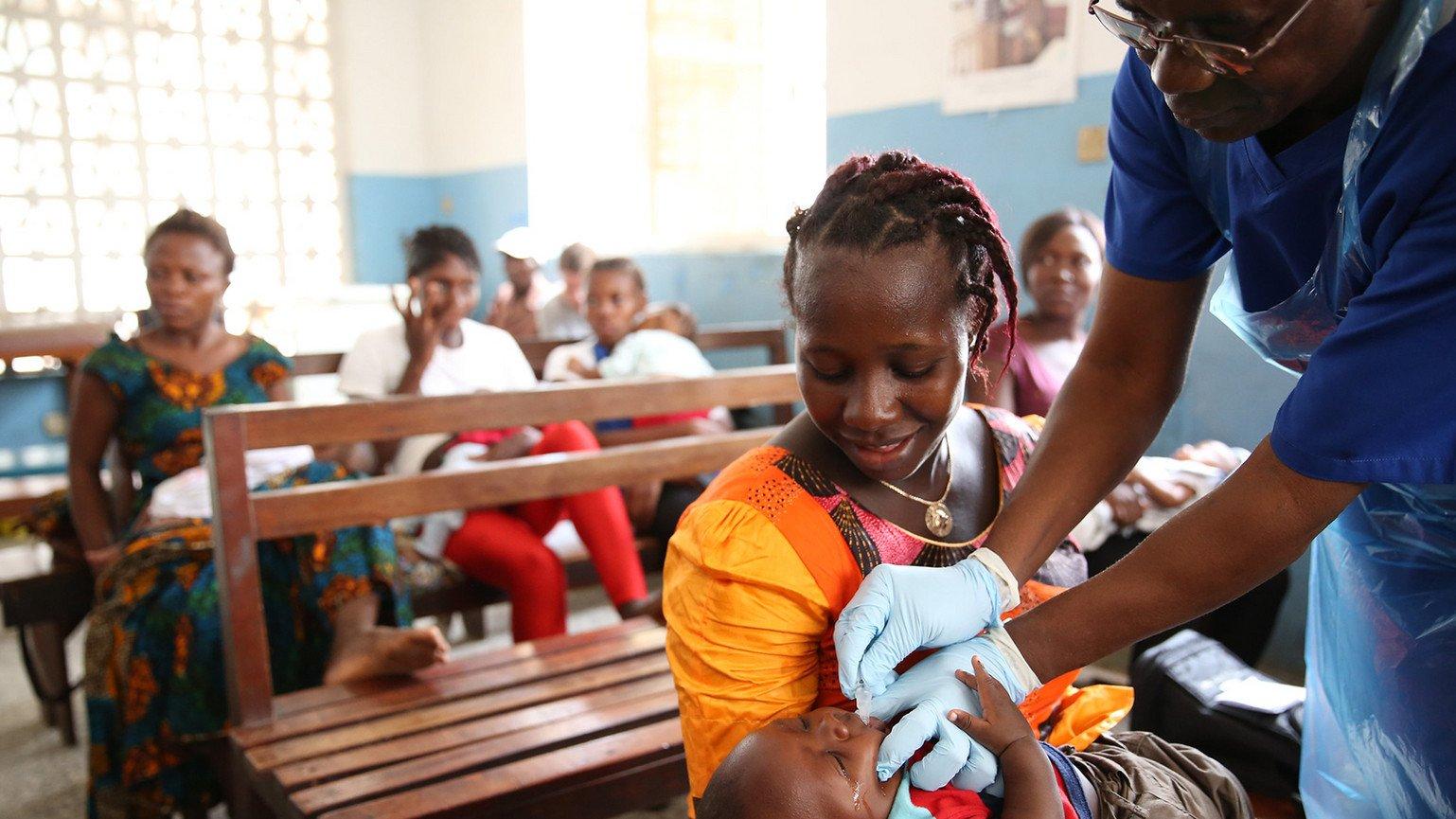
This week the European Medicines Agency (EMA) approved licensure of the Merck Ebola Vaccine (Ervebo).
In 2014-2015, Wellcome supported the clinical trials for safety and efficacy for Ervebo as part of a momentous global collaborative effort of researchers, governments, NGOs, companies and funders coming together to accelerate a vaccine for Ebola.
This was the start of my journey into epidemics, so I am very proud, humbled and excited to see that approval has been granted.
Licensure means that the EMA have reviewed the package of data on Ervebo collected during clinicals trials and deemed it meets the necessary efficacy and safety levels for use.
So, why does this matter?
If you think back to the West African Ebola outbreak of 2014, across three countries with over 28,000 suspected cases and 11,000 deaths – we had no vaccines, no treatments, no nothing. Just five short years later we have an approved and safe vaccine, something that many people said would never have been possible (vaccine development traditionally takes twice as long and can be up to 20 years).
The speed at which the global health community has pulled together has been remarkable and ultimately means that the future responses to Ebola can be quicker and simpler, with more lives protected and saved.
What does this mean for those fighting the disease on the ground?
Approved licensure of Ervebo provides further relief and support for patients, the field and healthcare workers and leaders in communities threated by Ebola.
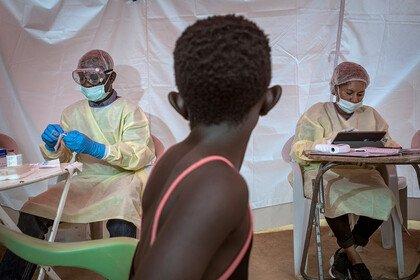
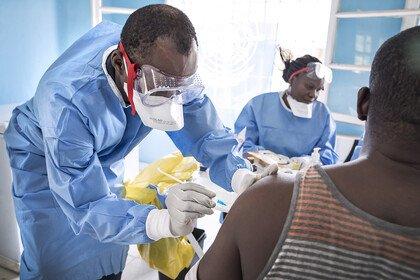
In the ongoing outbreak in the Democratic Republic of the Congo (DRC), many people continue to put themselves in high risk situations to ensure that communities who need the vaccine are able to get it. Further complexity has come from the unsettled and potentially violent situation in North Kivu. Add to this the need for lengthy patient consultation including explanation of consent forms, patient history and extensive examination on who patients may have been in contact with – required as the unlicensed vaccine was used under ‘compassionate use’ according to a ring vaccination strategy.
Ervebo licensure means it can be given with less process, paperwork and burden on patients or those trying to help them. And it can now be considered more flexibly by the leaders of countries at risk from Ebola outbreaks as they plan how best to protect their communities and health care workers.
What can this success teach us as we work to take on other epidemics?
- Integrating research into an outbreak response, rather than as an afterthought, is critical. Some research can only be done within an outbreak, for example assessing whether vaccines or drugs are effective. As it is not always clear when and where an outbreak will occur, ensuring that research protocols are pre-prepared and ready to use with collaborations in place, can collectively enable a quicker response. Ideally studies would be continuous, multi-year, multi-country and multi-epidemic.
- Building capability and expertise in areas that are likely to have epidemics and creating trust in the communities are key in containing outbreaks and saving lives. This new vaccine helps us in achieving that. We must enable countries at risk from epidemics to have access to the information and advice to make decisions on which tools and approaches make sense for their people.
- We must recognise epidemics as a continuum rather than discrete episodes. This requires sustained and ongoing efforts to address them rather than ad-hoc, reactive, crisis support. Only by doing this will we be able to prevent and mount effective responses to Ebola, Lassa, Nipah and the other diseases with epidemic potential.
- The power of partnership is key. For Ervebo, an unprecedented global partnership came together including the Government of Guinea, Médecins Sans Frontières, World Health Organization (WHO), UK Medical Research Council, Department for International Development, Norwegian Institute of Public Health, Merck and Wellcome to support the ebola vaccine in 2014-2016. This was all made possible by initial work on the vaccine by the Public Health Agency of Canada. We would not be where we are now without each of these partners and many others, working closely together to achieve shared objectives.
- We will be better prepared for the next epidemic due to the creation of the Coalition for Epidemic Preparedness Innovation (CEPI). CEPI was established in 2017. The founding partners, the Governments of Germany, Japan, Norway, the WHO, The Gates Foundation and Wellcome, collaborated to finance and coordinate vaccine research and development to ensure that vaccines were available against epidemic diseases.
- There is still more to do for Ebola vaccines and beyond. Complementing Ervebo with a second preventative vaccine is critical. Today in DRC, a Johnson & Johnson vaccine clinical trial was announced. We should also celebrate the early results that two Ebola drugs increase survival rates in Ebola-infected patients (a partnership between DRC, National Institute of Health, ALIMA, MSF, WHO and others). Let us use this momentum to focus efforts on development of much needed diagnostics and integration of multiple areas including social sciences into epidemic response, to get the job done for Ebola.
Epidemics of Ebola are inevitable, but the licensing of the vaccine by the EMA is a first step in being better prepared to prevent and control future Ebola outbreaks. It also shows how far we can come when multiple sectors come together to address a collective goal.
We can learn and do things better during the next epidemic, but for now let’s celebrate this momentous event!