Developing a vaccine for Ebola – the case for coordination and preparedness
The global community showed the power of working together during the West African Ebola epidemic. Continuing to do so could be key to improving our future epidemic preparedness.
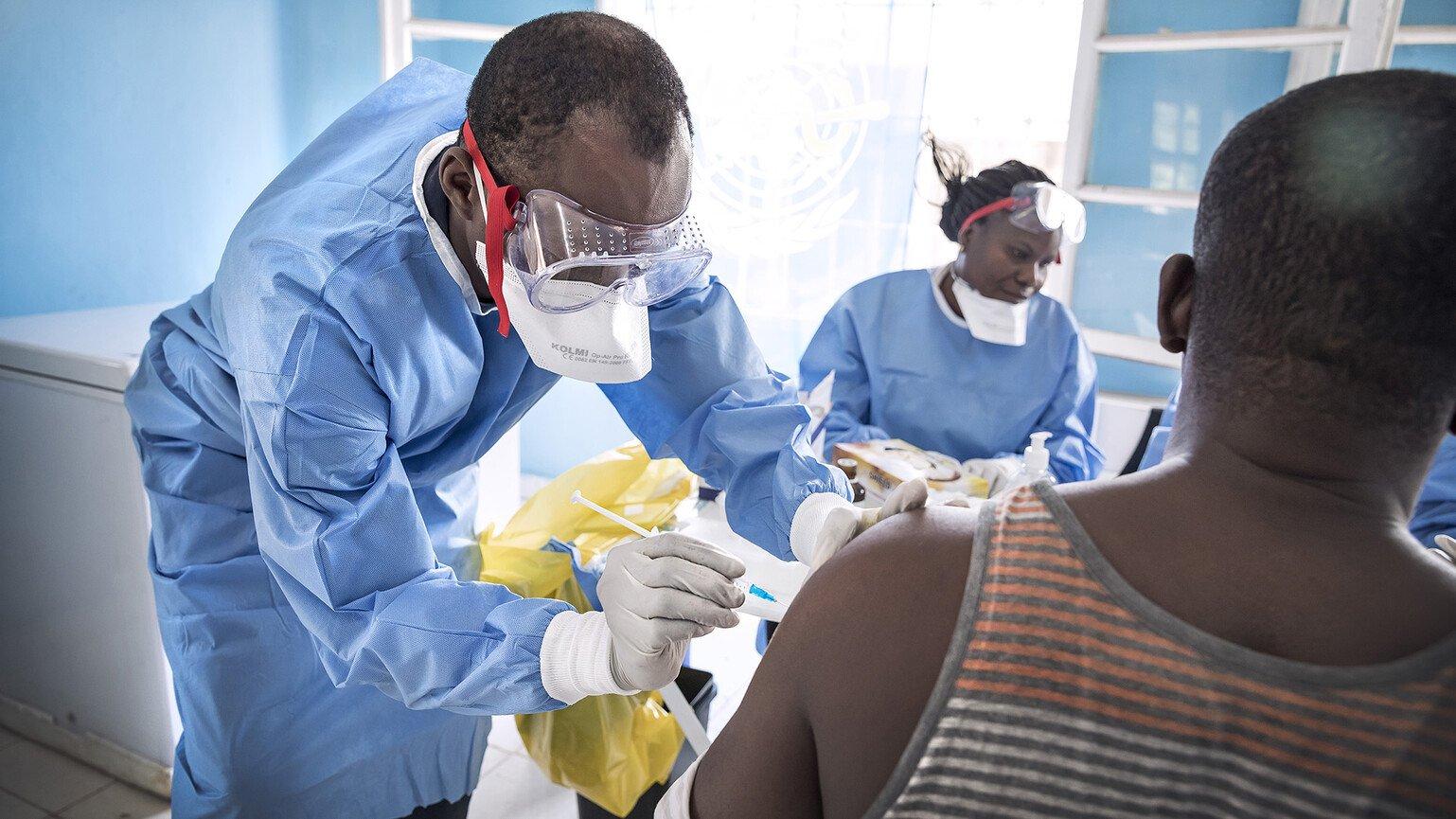
The first research showing that VSV-ZEBOV vaccine could help protect against Ebola brought sighs of relief. It was an incredible and humbling achievement that had only been possible due to the global collaboration of researchers, NGOs, governments, industry and funders – all working towards a unified goal.
The research, in July 2015, included data from approximately 7,500 people. It suggested that a single injection of VSV-ZEBOV might be highly effective in preventing people from contracting Ebola.
Already, the Ebola outbreak had taken thousands of lives and had a devastating impact across West Africa.
The VSV-ZEBOV news was positive for two reasons. Not only did this vaccine candidate appear to protect against contracting the Zaire strain of Ebola, but it had only taken about eight months to get to this point, rather than many years.
How the Ebola VSV-ZEBOV vaccine was developed
It was good coordination that accelerated the vaccine development process. But it involved starting from scratch and building new relationships between different organisations and with the local communities, to consolidate trust and share information effectively.
Promising Ebola vaccine candidates existed, but only one had been tested in humans and it was subsequently abandoned. When the Ebola outbreak started in West Africa in 2013 there were no vaccines ready to be tested.
Determining whether a vaccine is safe for human use and offers protection against a disease usually takes more than two years. The alarming scale of the outbreak meant this process needed to speed up if there was to be any hope of developing a vaccine in time to help bring the epidemic to an end.
The global community faced an enormous challenge – and time was not on our side.
In August 2014, Wellcome created an Ebola research fund and invited applications from researchers doing clinical trials to test Ebola vaccines and drugs. The first applications arrived within days of our funding announcement.
It took hard work by many people, including applicants, reviewers and Wellcome staff, to fast-track Ebola research funding.
One of the applications was a proposal to assess the safety of the Ebola vaccine VSV-ZEBOV, originally developed by the Health Agency of Canada.
By the end of October 2014 healthy volunteers were being vaccinated to assess the vaccine’s safety. The trial sites were in Europe (Germany and Switzerland) and Africa (Gabon and Kenya).
In parallel, with the MRC and Department for International Development, we co-funded the safety trial of another promising Ebola vaccine, ChAd3. This work was led by Professor Adrian Hill at the University of Oxford.
As the epidemic progressed, and with positive results from the safety trial, in December 2014 we received a proposal to assess whether VSV-ZEBOV could protect people from Ebola.
The researchers wanted to use an innovative trial design, where family, friends and contacts in a 'ring' around a patient with Ebola would be given the vaccine.
In March 2015, ring vaccination began in Guinea. It involved 11,841 people; among the 5,837 people vaccinated, no Ebola cases were recorded 10 days or more after vaccination.
The trial was led by partners including the Guinean authorities, World Health Organization (WHO), Médecins Sans Frontières and the Norwegian Institute of Public Health.
The vaccine is under ‘compassionate use’ – because it is still being licensed – in the current Ebola outbreaks in the Democratic Republic of the Congo.
What we’ve learned about preparing for future epidemics
When the West African Ebola outbreak occurred, the global community was not prepared. There were no vaccines, no treatments, few diagnostics, too few medical teams and no plan of action for who needed to do what.
While the healthcare community deserves credit for fast-tracking an Ebola vaccine, the vaccine was not available in time to stop the outbreak.
Think what could have been achieved if VSV-ZEBOV had been assessed for safety straight after the initial preclinical work.
If the safety work had been done before the outbreak crisis, the vaccine's efficacy could have been assessed much sooner, perhaps changing the course of the epidemic and ultimately saving lives.
This transition from preclinical to safety clinical trials is a sticking point in the vaccine development pathway for many infectious diseases, not just Ebola. The global community has not previously been willing or able to invest in the complex and costly development process required to develop new vaccines.
The West African Ebola outbreak, unprecedented in its scale and impact, emphasised the need to improve epidemic preparedness. There were two major outcomes.
- WHO developed an R&D blueprint for action to prevent epidemics. It sets out a list of priority pathogens for which we urgently need to develop vaccines and treatments. And it also sets out principles for global collaboration to prevent future epidemics.
- The Coalition for Epidemic Preparedness Innovation (CEPI) was formed in response to expert reports into the Ebola outbreak that called for a new system to stimulate vaccine development. A collaboration between government, philanthropy, industry and civil society, CEPI’s mandate is to finance and coordinate vaccine development to help contain outbreaks before they become emergencies.
Together with the collective and individual lessons we learnt, these two major initiatives place the global community in a better place to tackle future epidemic challenges.
But if we want to reduce the risk of future epidemics further, we need to amplify coordination to a new level. This means continuing to build relationships across sectors, sharing information more effectively, and working together transparently to outsmart epidemics.
